
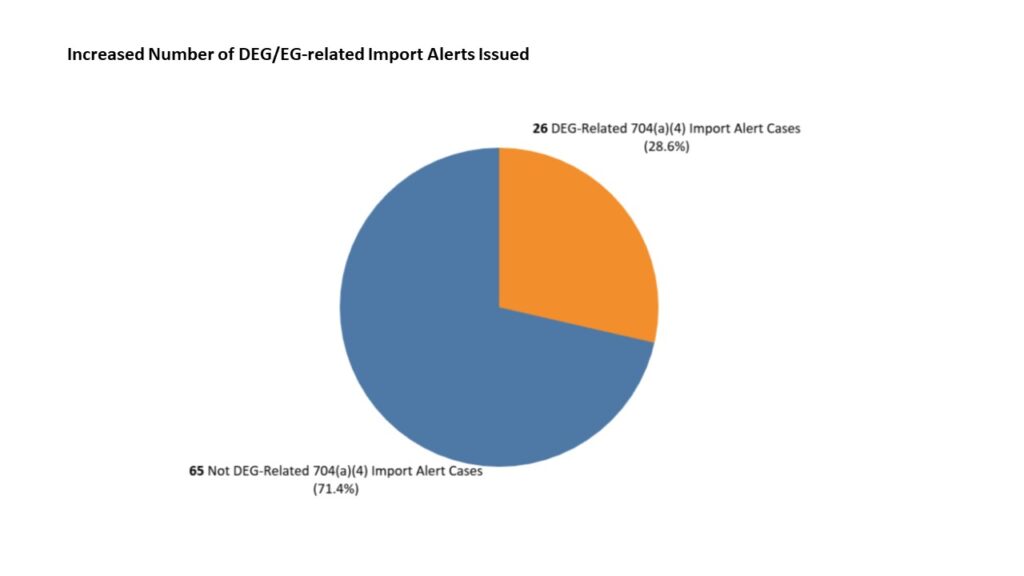
Impurities have become a significant concern for the industry, particularly following recent discoveries of harmful contaminants in widely used drugs. The presence of Diethylene Glycol (DEG) and Ethylene Glycol (EG) in medicinal products has emerged as a critical issue, leading to global recalls and triggering more stringent regulations by agencies like the U.S. FDA. These impurities, often resulting from inadequate oversight or cost-cutting measures, have spurred the industry to refine its impurity profiling techniques to protect public safety.
As impurity profiling becomes increasingly vital to maintaining drug safety, chromatographic techniques have risen to the forefront as an essential tool for identifying and quantifying these dangerous compounds.
The upcoming 2nd Annual Pharma Impurity Conclave 2024 in Hyderabad is set to address these developments, providing a platform for discussions about impurity profiling, the role of advanced chromatographic methods, and the need for stronger regulatory compliance.
The Danger of DEG and EG

The contamination of drugs with DEG and EG has resulted in numerous public health crises over the past decades. Both substances are toxic and have been involved in several incidents, including mass poisonings. Most notably, the contamination of cough syrups with DEG in countries such as India and Nigeria resulted in tragic deaths, especially among children. DEG is often found as a contaminant when it is mistakenly used as a cheaper substitute for non-toxic solvents like glycerin or propylene glycol during drug manufacturing.
The most recent case involving DEG contamination surfaced in 2023, when cough syrups exported from India were found to contain dangerously high levels of DEG and EG. This discovery led to international outcry, with the WHO issuing alerts and multiple countries recalling the products. These incidents underscore the importance of rigorous impurity testing and profiling during the manufacturing process, as even minor oversights can have devastating consequences for patients and public trust in pharmaceuticals.
Impurity Profiling and the Role of Chromatography
The incidents of DEG and EG contamination have highlighted the importance of impurity profiling—an analytical process that ensures all potential impurities are identified and quantified within a drug product. Impurity profiling involves a comprehensive approach to understanding the chemical makeup of a compound and detecting any unwanted substances that may compromise safety or efficacy.
Chromatography, particularly Liquid Chromatography (LC) and Gas Chromatography (GC), plays a pivotal role in impurity profiling. These techniques allow for the precise separation, identification, and quantification of impurities within complex pharmaceutical mixtures. For example, chromatographic methods can detect even trace amounts of DEG and EG in drug formulations, ensuring that manufacturers adhere to safety standards and prevent toxic contaminants from reaching the market.
With growing regulatory scrutiny, manufacturers are under increasing pressure to implement robust impurity profiling protocols. This means investing in advanced chromatography systems that can detect a wider range of impurities with greater sensitivity. High-Performance Liquid Chromatography (HPLC) and Ultra-High-Performance Liquid Chromatography (UHPLC) are among the most effective tools currently used for this purpose, offering precise impurity detection that complies with stringent global standards.
FDA’s Stringent Guidelines: Pushing the Industry Forward
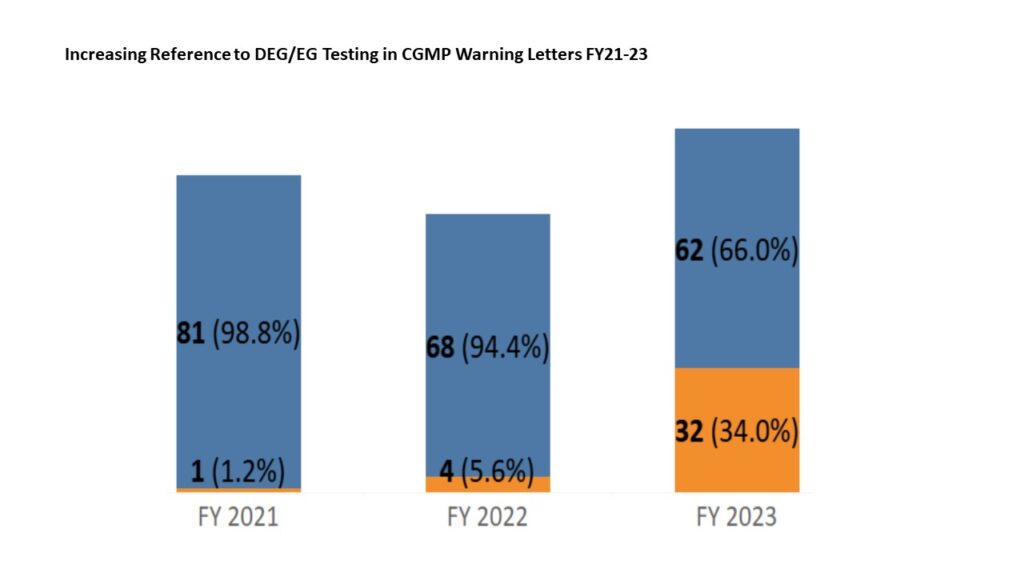
In response to contamination incidents, the U.S. FDA has intensified its guidelines and oversight regarding impurities in pharmaceutical products. The agency now requires drug manufacturers to conduct detailed impurity profiling, particularly focusing on known toxic substances like DEG and EG. These guidelines emphasize the importance of thorough testing at every stage of the manufacturing process, from raw materials to final products.
The FDA’s efforts have pushed the industry to adopt stricter quality control measures, encouraging companies to adopt better technologies for impurity detection. For instance, manufacturers are now required to conduct comprehensive risk assessments to evaluate potential sources of contamination and ensure that all ingredients meet purity specifications. In addition, the FDA has established clear limits for acceptable levels of impurities, compelling companies to stay within these parameters to avoid penalties, recalls, or product bans.
The FDA’s vigilance and the threat of severe consequences have made compliance a top priority for pharmaceutical companies worldwide. Manufacturers must now focus on continuous monitoring and testing to ensure they are meeting regulatory requirements and maintaining the highest standards of quality.
Pharma Impurity Conclave 2024: A Platform for Solutions
With incidents of contamination still fresh in the industry’s collective memory, the 2nd Annual Pharma Impurity Conclave 2024 comes at a critical time. Set to take place in Hyderabad, the Conclave will serve as a key forum for addressing the challenges associated with impurity profiling, including the detection and control of DEG, EG, and other harmful contaminants.
Industry experts, regulatory officials, and leading technologists will gather to share their insights on advanced chromatographic techniques and best practices for impurity profiling. The event will also feature case studies and workshops demonstrating the importance of staying ahead of emerging impurity risks while maintaining compliance with the FDA’s stringent guidelines.
Conclusion
As the industry grapples with rising impurity challenges, impurity profiling has become a non-negotiable aspect of drug manufacturing. The contamination of pharmaceutical products with harmful substances like DEG and EG has resulted in tighter regulatory scrutiny and a renewed emphasis on quality control. Chromatography remains a vital tool in this battle, offering the precision needed to detect and mitigate these risks.
The Pharma Impurity Conclave 2024 will provide an invaluable opportunity for stakeholders to discuss the latest advancements in impurity profiling and chart a path forward in ensuring the safety and quality of products. By adopting cutting-edge technologies and adhering to stringent regulatory guidelines, the industry can safeguard public health and restore confidence in the medicines we all rely on.
————–
Vrushali Negandhi
Head: Research & Production
Eminence Business Media

