
Background
In 2002, US-FDA launched an initiative, ‘‘Pharmaceutical CGMPs for the 21st Century—A Risk-Based Approach,’’ to enhance and modernize the regulation of pharmaceutical manufacturing and product quality.1 One objective, among others, was to facilitate the implementation of a modern, Risk-based Pharmaceutical Quality Assessment system. The desired state has been described as a maximally efficient, agile, flexible pharmaceutical manufacturing sector that reliably produces high- quality drug products without extensive regulatory oversight. There has been significant progress toward this vision as evidenced by FDA programs and initiatives in such areas as pharmaceutical development and Quality by Design (QbD), Quality Risk Management (QRM) and Pharmaceutical Quality Systems (PQS), Process Validation, and emerging technologies. These programs and initiatives promote use of the best pharmaceutical science and engineering principles throughout the product life cycle. Another example is the FDA Quality Metrics Program, described in the November 2016 revised draft guidance for industry ‘‘Submission of Quality Metrics Data’’ (81 FR 85226)4. In June 2018, FDA initiated two voluntary programs that sought additional industry input on quality metrics. FDA solicited industry participation for a Site Visit Program (83 FR 30751)4 for manufacturing establishments to present the advantages and challenges associated with implementing and managing a Quality Metrics program, and for a Quality Metrics Feedback Program (83 FR 30748)4 to engage stakeholders in identifying mutually useful and objective quality metrics.
FDA continues to develop Quality Metrics Program but recognizes that Quality Metrics are only one element within a manufacturer’s larger effort to increase the maturity of their quality management system. Manufacturers that demonstrate QMM operate under an enhanced Quality Management System (QMS) that exceeds the minimum standards specified in CGMP regulations and focuses on continual improvement.
Characteristics of a mature QMS include, for example, the ability to consistently and reliably deliver quality product over time, operational stability, and a strong quality culture. Additionally, for manufacturers with a mature QMS, FDA can exercise a more flexible regulatory approach, leading toward the goal of producing high-quality drug products without extensive regulatory oversight.
A transparent method of evaluating and communicating QMM is needed to fully realize the 21st century pharmaceutical quality vision. Toward that end, FDA initiated the QMM Finished Dosage Form (FDF) Pilot Program. Through this pilot program, a third-party contractor identified by FDA to conduct an assessment of a manufacturer’s QMS, accompanied by FDA staff. FDA will gain insight from the results of QMM assessments to inform the development of a rating system to measure and rate QMM.
Assessments under the QMM FDF Pilot Program will cover multiple topics.
Examples include but are not limited to:
- Supply chain management;
- Manufacturing strategy and Operations;
- Safety, environmental, and regulatory compliance;
- Inventory management;
- Performance management and continual improvement;
- Risk management;
- Management review and responsibility;
- Planning;
- Workforce management;
- Quality culture; and
- Customer experience.
 In the same timeframe as the QMM FDF Pilot Program, FDA will conduct a QMM pilot program for foreign manufacturers of Active Pharmaceutical Ingredients (APIs), including facilities manufacturing drug substance intermediates used to produce APIs.
In the same timeframe as the QMM FDF Pilot Program, FDA will conduct a QMM pilot program for foreign manufacturers of Active Pharmaceutical Ingredients (APIs), including facilities manufacturing drug substance intermediates used to produce APIs.
QMM program aims to encourage drug manufacturers to implement Quality Management practices that go beyond current good manufacturing practice (CGMP) requirements.
The QMM Goals:3
- Foster a strong quality culture mindset
- Recognize establishments that have advanced quality management practices and acknowledge establishments that strive to continually improve Quality Management practices
- Identify areas where Quality Management practices can be enhanced and provide suggestions for growth opportunities
- Minimize risks to product availability to assure reliable market supply
Adopting mature Quality Management practices supports a more reliable drug supply chain by reducing the occurrence of quality-related failures and improving the ability of establishments to maintain performance during expected and unexpected supply chain disruptions. Integrating business and manufacturing operations with quality practices and technological advancements can help achieve higher levels of maturity. This can optimize manufacturing process performance and product quality, enhance supply chain reliability, and foster proactive continual improvement.
Product Quality gives Manufacturer a confidence that every batch will be acceptable for release to market. Product Quality gives consumers a confidence, in every product dose they take.
This is important as Patients do not understand about Quality but the effectiveness of Quality medicines every time they take, are very safe & fit for the purpose.
QMM helps to ensure that there are no drug shortages due to Quality & Manufacturing issues.
 My take on the term QMM is, I: “Quality Management” & II: “Management Maturity” which will ensure Culture of Quality & concept of “Everyone is responsible for Quality”, to minimise the serious Quality & Manufacturing issues effectively. This will help in converting QMS to QMM.
My take on the term QMM is, I: “Quality Management” & II: “Management Maturity” which will ensure Culture of Quality & concept of “Everyone is responsible for Quality”, to minimise the serious Quality & Manufacturing issues effectively. This will help in converting QMS to QMM.
The Quality assessment of any drug, marketing or licensing application, includes assessment of the drug substance and drug product, as well as the proposed manufacturing process, facilities, and control strategy. Pharmaceutical Quality is achieved by assuring every dose of a drug on the market is safe, effective, and free of contamination and defects.
All sites manufacturing a drug must adhere to CGMP requirements, which define the minimum manufacturing standards to legally market drug products in USA. Compliance with CGMP requirements assures proper design, monitoring, and controls for manufacturing processes and facilities. FDA facility evaluation and surveillance, including facility inspections, provide assurance that sites manufacturing for the U.S. market comply with CGMP. Together, this regulatory oversight provides U.S. patients and consumers’ confidence in every dose of medicine they receive FDA regularly evaluates manufacturing facilities and takes action, if needed, to enforce CGMP regulations. FDA investigators look for deficiencies in meeting CGMP, but these evaluations do not measure how far a site’s PQS rises above the minimum requirements. Simple adherence to CGMP standards does not indicate, for example, that a firm is investing in improvements to prevent supply disruptions. The ICH Q10 PQS guidance augments CGMP with the concept of an effective PQS over the lifecycle of a product. ICH Q10 describes activities to manage and continually improve the PQS (the elements), using knowledge management and quality risk management principles (the enablers).1
Main reason which made US FDA to initiate QMM was Drug shortages issue affecting the patients lives. FDA report 2019 observed that main reason for 62% of drugs that went into shortage between 2013 and 2017 were associated with manufacturing or product quality problems (e.g., substandard manufacturing facilities/processes or quality defects in the finished product). This scenario was improved further in 2022-23 where FDA observed reduction in “Manufacturing Quality” related issues, reducing further drug shortages to 40% 1, 2. Hence US FDA decided to take further steps to minimise such issues & started educating The Pharma Industry on “Quality Management Maturity” (QMM).
The FDA has long held a vision for pharmaceutical quality in the 21st century, which has been described as “a maximally efficient, agile, flexible manufacturing sector that reliably produces high-quality drug products without extensive regulatory oversight”
The Drug Shortages Task Force proposed three enduring solutions to the problem of drug shortages; one solution was developing a rating system to incentivize drug manufacturers to invest in achieving QMM. QMM is the state attained by having consistent, reliable, and robust business processes to achieve quality objectives and promote continual improvement. Gauging QMM requires, in part, determining how well and how thoroughly a manufacturer has implemented the concepts of ICH Q10 (Figure 1)3.
A transparent rating system could inform purchasers about the level of QMM at sites from which they purchase drugs. In the absence of the transparency generated by ratings of QMM, there is risk that price competition and cost minimisation will continue to be key market drivers, especially for generic drugs, without direct reward for manufacturers who actively invest to avoid future shortage.
Some pharmaceutical firms have been slow to implement robust, mature quality systems and adopt the quantitative measures of quality that have been successful in the automotive and aerospace industries.2
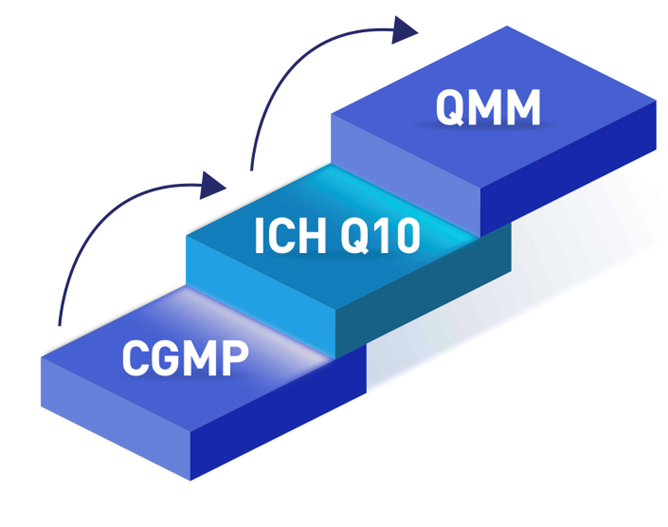
CGMP requirements define the minimum manufacturing standards to legally market drug products in the US. The ICH Q10 Pharmaceutical Quality System guidance augments CGMP with the concept of an effective pharmaceutical quality system over the lifecycle of a product. QMM requires, in part, thoroughly implementing the concepts of ICH Q10 to promote continual improvement.
FDA has conducted two pilot programs with pharmaceutical manufacturers to inform the criteria used to objectively measure a manufacturing site’s QMM. One pilot is focused on domestic manufacturers of FDF products, and the other on foreign manufacturers of APIs.
Feedback from the participants in the pilot programs is now helping determine best practices for conducting the assessments, the assessment tool, and associated logistics.
Minimizing the burden on manufacturing sites during QMM evaluations is an important consideration in the development of a QMM program.
QMM ratings are a part of an evolution toward performance-based regulatory practice and, as such, they may raise concerns from some. Public transparency is often a necessary driver for industry improvement.
The long-term effects of an FDA QMM program could be far-reaching:
Transparency in the market could provide top-rated manufacturers in the U.S., both large and small, with a competitive advantage, potentially enabling them to grow market share and increase their workforce. Manufacturers with higher QMM focus on continual improvement and are therefore more likely to embrace advanced manufacturing technologies which can improve the capability and robustness of the industry and lead to an expansion of domestic pharmaceutical manufacturing.
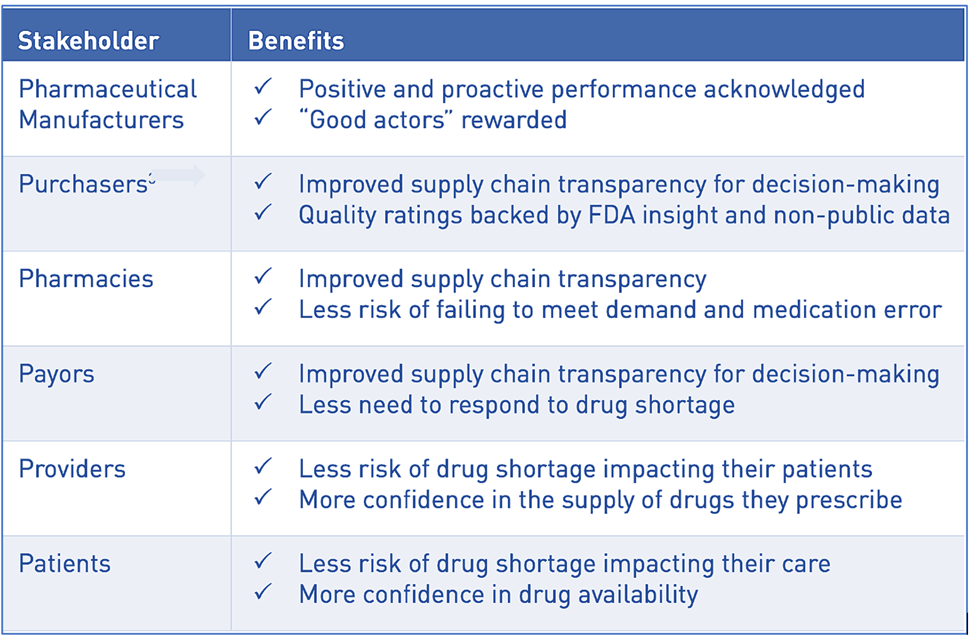
The potential benefits for stakeholders are clear: manufacturers with higher QMM get recognition in the market; purchasers and payors get more insight and confidence into the supply chain of the drugs or components they buy or reimburse; and patients, pharmacies, and healthcare professionals get medicine less at risk of shortage. Everyone will have more confidence in the next dose of medicine.
QMM is NOT an additional burden or requirement. It is, in fact, integral to an establishment’s quality system. Most establishments already have processes and practices aligned with QMM. Achieving higher levels of QMM naturally results from an establishment’s proactive continual improvement efforts. Investing in a culture of quality should not require the creation of new departments; a quality culture should be integral to the quality system.
Conclusion
QMM represents a transformative approach to quality assurance in the pharmaceutical industry. By fostering a culture of excellence, continuous improvement, and proactive risk management, QMM enhances product reliability, reduces operational risks, and safeguards public health. The USFDA’s efforts to promote QMM are instrumental in driving industry-wide adoption, ensuring a sustainable and resilient Pharmaceutical Supply Chain.
The QMM program seeks to promote a strong culture of quality, recognize establishments with robust Quality Management Practices, and provide support and recommendations for areas where Quality Management Practices can be enhanced. Through the program, industry participants and QMM assessors can work together to drive proactive continual improvement in the pharmaceutical industry
 With the right incentives, technological advancements, and global harmonization, QMM has the potential to become a cornerstone of Pharmaceutical Quality Management, benefiting patients and the healthcare system for years to come.
With the right incentives, technological advancements, and global harmonization, QMM has the potential to become a cornerstone of Pharmaceutical Quality Management, benefiting patients and the healthcare system for years to come.
In Part II, we will discuss the Strategy for Quality Management Maturity Assessment Process / measures.
References:
1: FDA. Drug Shortages: Root Causes and Potential Solutions 2019 [Available from: https://www.fda.gov/media/131130/download.
2: Dr Jacquin Jones U.S. FDA India Office – International Relations Specialist 27-28 June 2024 IPA’s 9th Global Pharmaceutical Quality Summit
3: CDER’s Office of Pharmaceutical Quality White Paper on Quality Management Maturity (April 7, 2022)
4: Federal Register / Vol. 85, No. 201 / Friday, October 16, 2020 / Notices
5: FDA. Guidance for Industry: Q10 Pharmaceutical Quality System 2009
6: FDA Guidance for Industry: Q9 Quality Risk Management
7: FDA Guidance for Industry: Q12 Life Cycle Management

