
Jegan Israel
General Manager Quality & Compliance
Zydus Lifesciences
Every life science company knows that quality is of the utmost importance. But defining quality and aligning that definition to regulations requires an intentional approach and this is how QMS came into the picture. QMS; Quality Management System, a comprehensive system that a an organization uses to manage quality throughout its operations.
Structure of QMS is much like a pyramid, the pinnacle document that simply and elegantly defines the goals of the QMS is the quality policy. Here are the 9 core elements of a well-defined Quality Management System:
1. Quality Policy
The top-level documents for any QMS are the quality policy, quality manual, and quality objectives. When you begin creating a quality management system, the first step should be to draft a quality policy.
2. Quality Manual
A quality manual is an overview of the entire QMS that can be given to a customer or auditor to help them quickly understand how the QMS is structured and which QMS area, if any, the organization is exempt from or otherwise does not apply to their system.
3. Quality Objectives
These objectives are designed to encourage organizations to define strategic goals and a purpose for the QMS. Objectives translate an organization’s vision into practice by creating a link between customer requirements and specific, measurable, and attainable goals.
4. Organizational Structure and Responsibilities
The top-level documents provide a basic framework and starting point for the QMS, but they do not contain enough detail to ensure quality. A QMS needs various policies, procedures, processes, documents, and records to maintain consistent quality and document evidence of that quality.
5. Document Records Control and Management
In a QMS, all documents must be controlled and all records must be retained. Think of documents as procedures, form templates, the quality manual, work instructions, approved supplier lists, and other documents that contribute to making the product in any small way.
6. Processes and Procedures
Standards for quality management require organizations to identify and define all organizational processes that use any resource to transform inputs into outputs.
7. Data Management and Analysis
Data analysis should be used to identify processes or systems that are out of control as early as possible instead of waiting until major nonconformance occurs.
8. Continuous Improvement
Maintaining quality and process performance at consistent levels is the most basic goal of any QMS, but when fully implemented and mature, that QMS should allow for improvement of quality and processes.
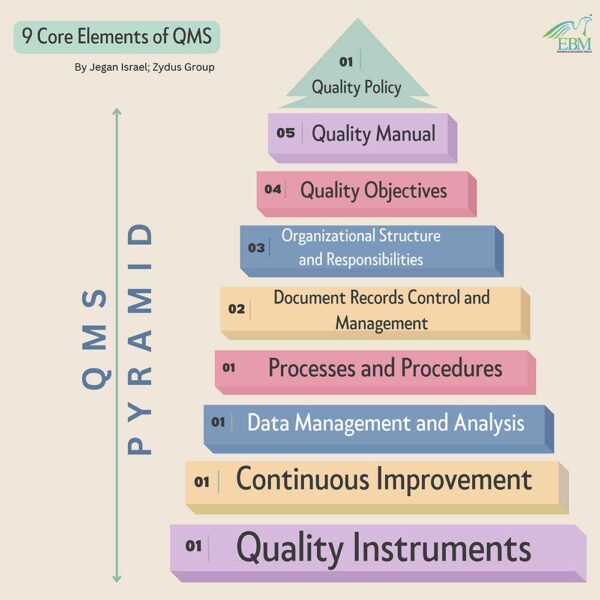
9. Quality Instruments
The success of a QMS hinges on the proper control and calibration of tools utilized for measuring quality, ensuring it is crucial for the effectiveness of a QMS.
In a nutshell, a robust Quality Management System (QMS) is vital for life science companies to ensure regulatory compliance and uphold high-quality standards. The QMS, structured like a pyramid, includes key elements such as the quality policy, quality manual, and quality objectives. The quality policy sets the foundation for the QMS, while the quality manual offers an overview for stakeholders. Quality objectives drive strategic goals, linking customer requirements to measurable targets. Organizational structure defines responsibilities, and document control along with data management ensures accuracy and compliance. Continuous improvement is essential to the QMS’s success, supported by quality instruments within the overall quality framework.
—————————————————————

